925-200-4687
1978 W Winton Ave
Hayward, CA 94545
East Outer Fremont
OVERVIEW
Previously operated by Lonza, this turnkey multiproduct cGMP facility
is purpose-built for clinical-stage biopharmaceutical manufacturing specializing in the production of biotherapeutics, including antibodies, fusion proteins, and enzymes, using mammalian cell culture. The facility supports technology transfer, single-use manufacturing, and comprehensive analytical and regulatory services.
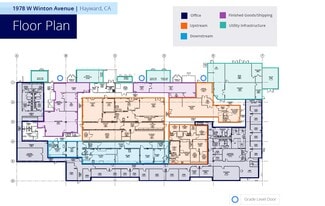
• Upstream Processing: power and utili-ties to operate 2 x 1,000L and 2 x 2,000L single-use bioreactors, enabling flexible, high-yield production.
• Downstream Processing: Robust purification capabilities to ensure product quality and compliance.
• Support Services: Includes pilot labs, analytical testing, stability studies, quality control, and regulatory support for seamless process development.
• Aseptic Filling: Single-use technology for bulk drug substance filling into bags and bottles, with frozen or refrigerated storage options.
• Infrastructure: Designed for 20/7 operations, offering scalability for future teams.
• Location Advantage: Strategically located in Hayward, CA, with proximity to Bay Area Life Sciences hubs
Existing Infrastructure:
• Power (1,000 amps @ 277/480v)
• Backup Generator (1,000 gallons)
• Connectivity for WFI system previously outfitted with 10kL HWFI storage/recir-culation tank and previously installed HWFI loop and POU heat exchangers to ambient WFI.
• New Chiller supporting cold rooms
• 3rd party bulk gas service (O2, CO2 and LN2)
• Upgraded Boilers
• Lift pump for waste to central neutralization system
RENTAL DETAILS
SPACE AVAILABLE
38,664 SF
# OF SPACES
1
RENT/MONTH
$115,992 USD /mo
RENT RATE (MO)
$3.00 USD /SF/mo
RENT RATE (YR)
$36.00 USD /SF/yr
LEASE TYPE
Triple Net
LEASE LENGTH
Negotiable
AVAILABLE
Now
BUILDING DETAILS
PROPERTY TYPE
Flex
PROPERTY SUBTYPE
R&D
TOTAL BUILDING SIZE
38,664 SF
YEAR BUILT
1970
UTILITIES
Water, Sewer
CLEAR CEILING HT
-
DOCKS
-
DRIVE INS
3
PARKING SPACES
60
LAND DETAILS
LAND ACRES
2.2 AC
LAND SF
95,832 SF
ZONING
-
ASK ABOUT THIS PROPERTY
Please correct the highlighted field(s).

925-200-4687
By clicking the button, you agree to Showcase's Terms of Use and Privacy Notice.
Please correct the highlighted field(s).

925-200-4687
By clicking the button, you agree to Showcase's Terms of Use and Privacy Notice.